
The change in entropy of a system for an arbitrary, reversible transition for which the temperature is not necessarily constant is defined by modifying. Stirling Engine The steps of a reversible Stirling engine are as follows.
Then it goes through the following steps:Entropy, S, is a state function and is a measure of disorder or randomness. A positive (+) entropy change means an increase in disorder. The universe tends toward increased entropy. All spontaneous change occurs with an increase in entropy of the universe.Which is the correct formula of entropy If the process is reversible, then the change in entropy is equal to the heat absorbed divided by the temperature of the reversible process. In the equation, Q is the heat absorbed, T is the temperature, and S is the entropy. Entropy is also the measure of energy not available to do work for your system.5.5 ENTROPY CHANGES OF AN IDEAL GAS For one mole or a unit mass of fluid undergoing a mechanically reversible process in a closed system, the first law, Eq.
Step AB: isothermal expansion at from to Enthalpy and Entropy are two significant terms related to thermodynamics. Step CD: isothermal compression at from to(a) Draw the pV diagram for the Stirling engine with proper labels.(c) How does the efficiency of the Stirling engine compare to the Carnot engine working within the same two heat reservoirs?Strategy Using the ideal gas law, calculate the pressure at each point so that they can be labeled on the pV diagram. Isothermal work is calculated using and an isochoric process has no work done.
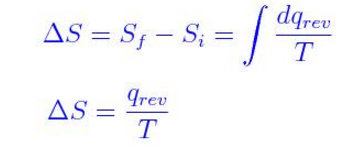
Hence, its own energy content gets low, according to the fundamental concept of energetics.It happens because during a chemical reaction, some bonds of reactions need to be broken to produce the product. The reason behind it is if a system participates in a reaction, it releases energy. Also, it is concluded that if the enthalpy decreases, a reaction is successful. The enthalpy is represented through the following equation.Where E is enthalpy, U is internal energy of any system, P is pressure, and V is volume.The change of enthalpy in a reaction is almost equivalent to the energy gained or lost during a reaction.


This term comes from Greek and means “a turning” point. This idea is derived from Thermodynamics, which explains the heat transfer mechanism in a system. Following is the example of such a reaction.CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(I) ΔH⁰ c = -890kJmol⁻✟rom the above equation, it is proved that, whatever compound is burned, has to take 1M of its heat energy only.Also, it is to be noted that, the standard enthalpy change of combustion for hydrogen is same as a change of formation of water.The Entropy is the measure of the disorder of the energy of a collection of particles. As burning always produces heat, the value of this change will be negative in all circumstances. That’s why the fractionIn equations has to be there on the left-hand side.This reaction shows that to form 1 M of liquid water, 286 kJ heat evolves.It happens when in the presence of oxygen, 1 M of any compound is completely burned.
Thereby the ice melts and entropy increases.Since all the spontaneous processes are irreversible, we can say that the entropy of the universe is increasing. For example, when we put a block of ice on a stove, these two make an integral part of a single isolated system. It states that any spontaneous change in an isolated system for irreversible reaction always leads towards the increasing entropy.
It is derived from the third law of thermodynamics. Also, it is expressed as Delta S, and following is the equation.Where S denotes the change in entropy, Q denotes reverse of heat and temperature is represented by T in Kelvin scale.It is a related term and is expressed by S. Due to this, it is said that the universe is “running down”.The S.I unit of entropy is Joules per Kelvin.
Change In Entropy Formula Free Energy Is
This relation is first stated in 1070’s by Josiah Willard Gibbs.It is expressed by G. This free energy is dependent on chemical reaction for doing useful work. If each configuration is probable equally, then the entropy is the natural logarithm of the total number of changes, multiplied by Boltzmann's constant.Where kB is Boltzmann's constant, S is entropy, ln is natural logarithm, and W denotes the number of possible states.Note: Boltzmann's constant= 1.38065 × 10 -23 J/K.Also, enthalpy entropy and free energy are closely related to each other as both entropy and enthalpy are combined into a single value by Gibbs free energy. In a system which can be presented by variables, they can predict a certain number of changes. The equation as followsFor a reversible thermodynamic process, Entropy can be expressed in calculus as an integral from the initial state of a process to its final state that is dQ/T. More specifically, entropy is a measure for probability and molecular randomness of a macroscopic entity.
We have a great collection of notes and model question on every chapter of chemistry be it physical, organic or inorganic.Hence, visit our website and start learning the fundamental topics of chemistry. For a spontaneous process, G is negative, and for a non-spontaneous process, G is positive.DIY: Find out the value of T from the enthalpy and entropy change for the reaction below.Where Delta H is 30 kJ mol -1 and Delta S is 105 J K mol -1If you want more information about enthalpy and entropy in Thermodynamics, check out our website or download Vedantu’s app on your Smartphone. If we subtract the product of T and S from Enthalpy, we get Gibbs free energy.The direction of a chemical reaction is determined by Delta G.


 0 kommentar(er)
0 kommentar(er)
